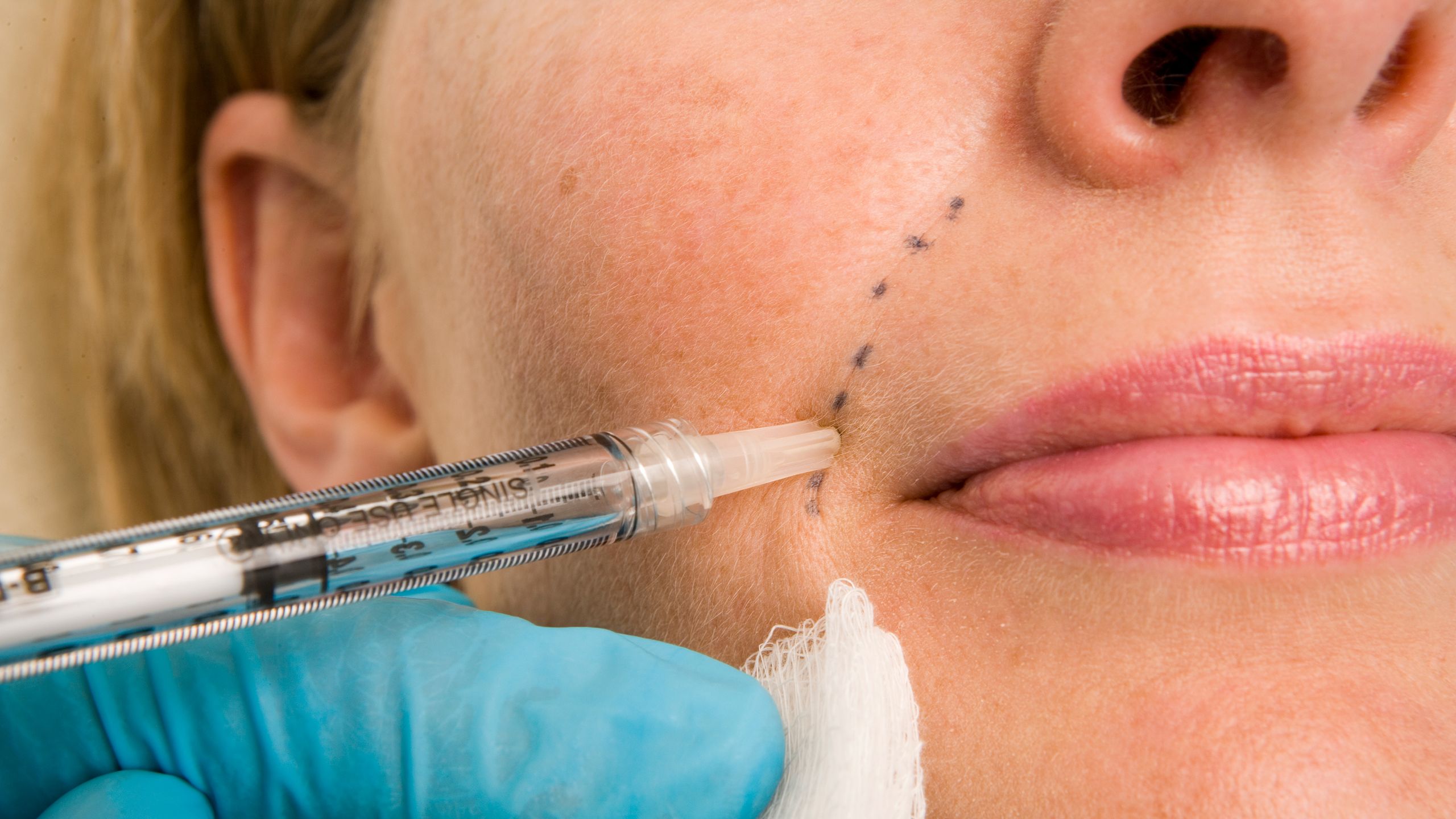
Have you ever considered getting a cosmetic injectable procedure done? Whether it’s for a touch of Botox or some filler, ensuring that your health practitioner is up to par with the latest guidelines is crucial. Recently, the Australian Health Practitioner Regulation Agency (AHPRA) introduced new guidelines for registered health practitioners performing non-surgical cosmetic procedures. These guidelines aim to bring more clarity and safety to the booming cosmetic medicine industry.
The new guidelines emphasize the importance of specific, approved training requirements for practitioners offering non-surgical cosmetic procedures. While these guidelines are a step in the right direction, some experts believe there is still room for improvement in ensuring patient safety and standardizing practices across the industry.
Dr. Susan O’Dwyer, Chair of the Medical Board of Australia, highlighted that these guidelines are essential to ensure all professions are
“working from similar playbooks”
when it comes to patient safety. She mentioned that practitioners need more than just foundational qualifications from their initial training to perform cosmetic procedures safely. Further education and training will be necessary for practitioners looking to expand their scope of practice.
According to AHPRA, practitioners should have
“appropriate education, knowledge, training, competence,”
and experience before performing any specific cosmetic procedure. This includes being able to manage any complications that may arise during or after the procedure.
While these guidelines set a clear expectation for practitioners, they do not prescribe a specific educational pathway. However, they stress the importance of adequate training in anatomy and physiology, assessing suitability for procedures, and hands-on training specific to the procedure being offered.
Moreover, all registered health practitioners involved in cosmetic procedures must undertake Continuous Professional Development (CPD) activities related to such procedures. This ongoing learning ensures that practitioners stay updated on best practices and advancements in the field.
One key aspect addressed by the new guidelines is prescribing practices for cosmetic injectables. The guidelines explicitly state that providing prescriptions via text, email, or online consultations is not acceptable practice. Instead, video telehealth or in-person consultations are recommended methods for prescribing injectables within regulatory boundaries.
Sheri-lee Knoop from the Cosmetic Nurses Association expressed both approval and concern regarding these new guidelines. She noted that while requiring appropriate education was positive progress towards ensuring safe practice within one’s scope of expertise; there was ambiguity surrounding what constitutes “appropriate” education.
The absence of formal recognition of non-surgical cosmetic treatments as a specialty presents challenges in data collection regarding issues or complications within this sector. Without standardized training pathways and clear definitions of roles within cosmetic medicine specialties like injectables can hinder effective regulation and oversight.
Dr. Lily Vrtik from Australasian Society of Aesthetic Plastic Surgeons emphasized how these new advertising reforms would help educate patients about potential risks associated with seemingly low-risk treatments like Botox injections. Standardizing training requirements remains an important focus area moving forward as it impacts patient safety outcomes significantly.
In conclusion, while these new guidelines aim to enhance safety standards in non-surgical cosmetic procedures by setting clear expectations for practitioners’ education and prescribing practices; further refinement might be needed based on expert feedback regarding standardization issues within this rapidly evolving field.






Leave feedback about this